How Is Lithium Extracted from Spodumene?
 Laura
Laura
 Apr 24, 2025
Apr 24, 2025
 1741
1741
If you want to know more details about equipment, solutions, etc, please click the button below for free consultation, or leave your requirements!

lithium mine
Lithium powers our modern world, from smartphones to electric vehicles (EVs). But before it reaches batteries, it’s locked inside minerals like spodumene, a lithium-rich ore found in hard rock deposits. Extracting lithium from spodumene is a complex but vital process. In this guide, we’ll down how spodumene is transformed into battery-grade lithium, step by step.
01 Why Spodumene?
BackSpodumene (LiAlSi₂O₆) is a top source of lithium due to:
High Lithium Content: Contains 6–7% lithium oxide (Li₂O), far richer than brine deposits.
Fast Processing: Unlike brine evaporation (which takes months), spodumene can be processed in days.
Purity: Yields high-grade lithium for premium batteries.

Spodumene ore
02 Steps to Spodumene Extraction
BackStep 1: Mining & Beneficiation
Before extraction begins, spodumene ore must be upgraded through beneficiation:
1). Crushing & Grinding:
Ore is blasted, crushed into gravel, then ground into a fine powder to liberate spodumene crystals.
2). Gravity Separation:
Heavy minerals like spodumene are separated from lighter waste using spirals or shaking tables.
3). Froth Flotation:
Chemicals: Collectors (e.g., fatty acids) make spodumene particles hydrophobic (water-repelling).
Air Bubbles: Carry spodumene to the surface as froth, while waste sinks.
Result: A spodumene concentrate with 5–7% Li₂O.
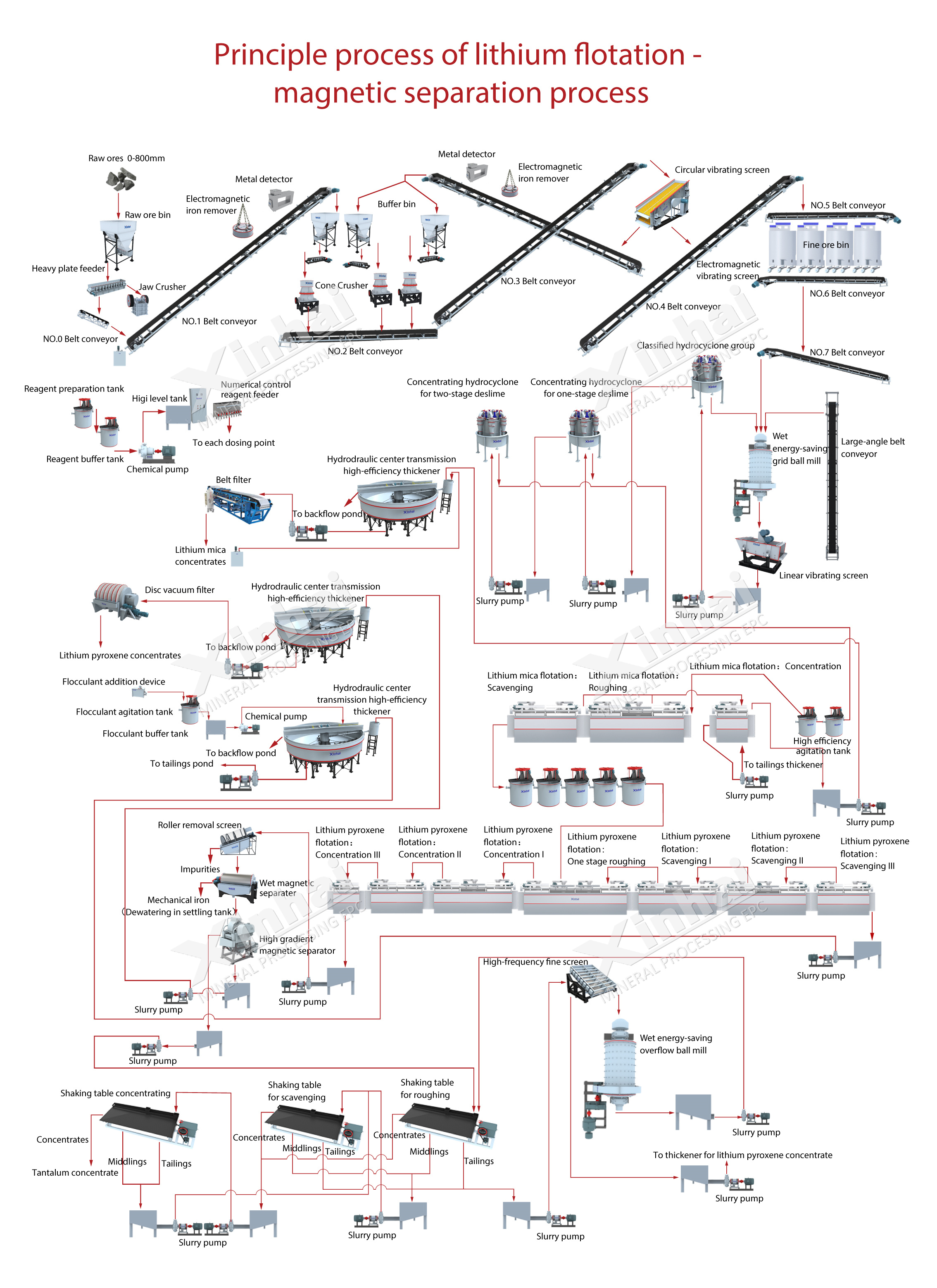
Lithium ore flotation-magnetic separation combined process principle flow chart
Step 2: Thermal Treatment (Calcination)
Raw spodumene is chemically stable and resistant to acids. To unlock lithium:
Heating: The concentrate is roasted at 1,100°C in a kiln.
Phase Change: Converts α-spodumene (stable) to β-spodumene (reactive), making it soluble in acid.
Step 3: Acid Leaching
The β-spodumene is mixed with sulfuric acid (H₂SO₄) to extract lithium:
Acid Digestion:
Heated to 250°C, β-spodumene reacts with acid to form lithium sulfate (Li₂SO₄).
Water Leaching:
The mixture is dissolved in water, creating a lithium sulfate solution.
Step 4: Purification & Precipitation
The lithium solution is cleaned to remove impurities:
Neutralization:
(Ca(OH)₂) is added to precipitate metals like iron and aluminum.
Filtration:
Impurities are filtered out, leaving a purified Li₂SO₄ solution.
Carbonation:
Sodium carbonate (Na₂CO₃) is added to precipitate lithium carbonate (Li₂CO₃), a white powder used in batteries.
Alternative: Adding sodium hydroxide (NaOH) produces lithium hydroxide (LiOH), preferred for high-nickel EV batteries.
Step 5: Drying & Packaging
The lithium carbonate or hydroxide is:
Dried: In rotary dryers to remove moisture.
Packaged: Shipped to battery manufacturers for further refining.

Construction of spodumene processing plant
03 Environmental & Economic Challenges
BackEnergy-Intensive: Calcination requires high heat, often from fossil fuels.
Tailings: Waste rock from beneficiation must be stored in lined ponds.
Acid Use: Sulfuric acid handling demands strict safety protocols.
04 Innovations in Spodumene Processing
BackDirect Lithium Extraction (DLE): Emerging tech skips calcination by using solvents or membranes.
Solar-Powered Kilns: Reduce carbon footprint of thermal treatment.
Recycling: Reusing process water and acids to cut waste.

Flotation equipment in a spodumene processing plant in Zimbabwe
05 FAQs
BackQ: Why not use lithium brine instead of spodumene?
A: Brine is cheaper but slower (12–18 months evaporation) and less pure. Spodumene meets booming EV battery demand faster.
Q: How much lithium is lost during processing?
A: Modern methods recover 80–90% of lithium from ore.
Q: Is spodumene mining sustainable?
A: While energy-heavy, new tech like DLE and renewables are making it greener.
06Conclusion
BackExtracting lithium from spodumene is a blend of geology, chemistry, and engineering. By upgrading ore through beneficiation, unlocking lithium with heat and acid, and refining it into battery-grade products, miners power the clean energy revolution. As demand for EVs soars, innovations in efficiency and sustainability will ensure spodumene remains a cornerstone of the lithium supply chain.
Feel free to contact us and learn more about lithium processing solutions!
 +86 182 3440 3483
+86 182 3440 3483 yanzhang19990421@gmail.com
yanzhang19990421@gmail.com



 Message
Message Chat Now
Chat Now

















