How Is Aluminium Extracted from Bauxite?
 Laura
Laura
 May 01, 2025
May 01, 2025
 2746
2746
If you want to know more details about equipment, solutions, etc, please click the button below for free consultation, or leave your requirements!

Bauxite ore
Aluminium is the most abundant metal in the Earth's crust, but it's never found pure in nature. Instead, it's locked inside bauxite ore—a reddish-brown rock rich in aluminium oxides. Extracting aluminium from bauxite involves a mix of physical and chemical processes. In this guide, we'll walk through how bauxite is transformed into shiny aluminium metal, focusing on the crucial beneficiation steps that prepare the ore for refining.
01Step 1: Mining Bauxite
BackBauxite is typically strip-mined in open pits, with major deposits in Australia, Guinea, and Brazil.
Location: Bauxite forms near the equator, where tropical weathering leaches silica from soil, leaving behind aluminium-rich ore.
Quality Check: High-grade bauxite contains 50–60% aluminium oxide (Al₂O₃). Low-grade ore requires more processing.

Bauxite ore
02Step 2: Crushing and Washing – Physical Beneficiation
BackRaw bauxite is mixed with clay, silica, and iron oxides. Beneficiation removes these impurities:
Crushing: Jaw crushers break bauxite into 10–20 cm chunks.
Washing: High-pressure water jets scrub away clay and silica.
Screening: Vibrating screens sort particles by size; finer material is sent for refining.
Why it matters: Washing boosts aluminium oxide content by 10–15%, saving energy in later stages.
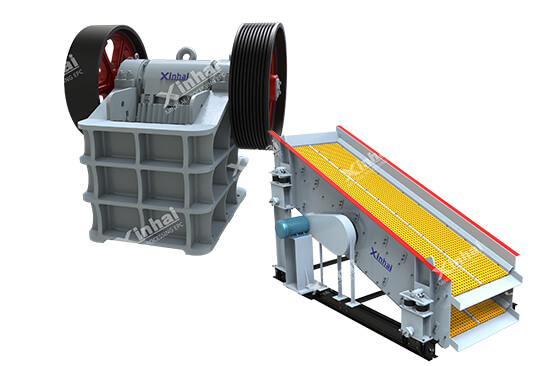
Crusher and vibrating screen
03Step 3: The Bayer Process – Chemical Refining
BackThe washed bauxite undergoes the Bayer process to extract pure aluminium oxide (alumina):
a. Digestion
Bauxite is mixed with hot sodium hydroxide (NaOH) at 150–250°C. This dissolves aluminium oxide into a sodium aluminate solution.
b. Clarification
The mixture is filtered to remove “red mud” (iron and silica waste). For every ton of alumina, 1–2 tons of red mud are produced.
c. Precipitation
The filtered solution is cooled, and pure alumina crystals (Al₂O₃) are precipitated out using seed particles.
d. Calcination
Alumina is heated to 1,000°C in rotary kilns, driving off water to create a white powder.
04Step 4: Smelting Alumina into Aluminium
BackAlumina is converted to metal via the Hall-Héroult process:
Electrolysis: Alumina is dissolved in molten cryolite (Na₃AlF₆) at 950°C.
Electric Current: Carbon anodes pass a current, splitting alumina into aluminium and oxygen.
Tapping: Molten aluminium sinks to the bottom and is siphoned off.
Fun Fact: Producing 1 ton of aluminium requires 14,000 kWh of electricity—enough to power a home for a year!
05Environmental Challenges
BackRed Mud: Storing this toxic byproduct safely is a major issue. Researchers are repurposing it into cement or rare-earth metal sources.
Energy Use: Smelting consumes 5% of global industrial electricity. Transitioning to renewables (e.g., hydropower in Canada) cuts emissions.
Land Impact: Restoring mined land with native plants prevents soil erosion.
06Innovations in Bauxite Beneficiation
BackDry Washing: New tech uses air instead of water to remove impurities, ideal for arid regions.
Bioleaching: Bacteria help break down silica, reducing chemical use.
Red Mud Recycling: Companies like Metallica Minerals extract titanium and iron from red mud.

Bauxite mine
07FAQs
BackQ: Why is bauxite used for aluminium production?
A: Bauxite has the highest aluminium content (30–60%) of any ore, making it cost-effective to refine
Q: Can aluminium be recycled from scrap?
A: Yes! Recycling uses 95% less energy than primary production.
Q: How much bauxite is needed to make 1 ton of aluminium?
A: Roughly 4–6 tons of bauxite → 2 tons of alumina → 1 ton of aluminium.
08Conclusion
BackExtracting aluminium from bauxite is a marvel of engineering, blending physical beneficiation with high-temperature chemistry. While the process is energy-intensive, innovations in recycling and red mud management are paving the way for greener aluminium. From soda cans to airplane parts, understanding this journey highlights why aluminium remains a cornerstone of modern life—and why sustainability is key to its future.
Feel free to contact us and learn more about bauxite processing solutions!
 +86 182 3440 3483
+86 182 3440 3483 yanzhang19990421@gmail.com
yanzhang19990421@gmail.com



 Message
Message Chat Now
Chat Now

















